Camber Launches 13 New Generic Products in Q1 2025, Sets Ambitious Pace for the Year
Camber is proud to announce the successful launch of 13 new generic products in the first quarter of 2025. With strong momentum, we’re on track to introduce over 60 new generic medications this year—continuing our mission to enhance accessibility and affordability across the healthcare landscape. In addition to these launches, Camber anticipates receiving 54 new ANDA approvals and plans to file 35 additional ANDAs in 2025, strengthening our already robust pipeline.
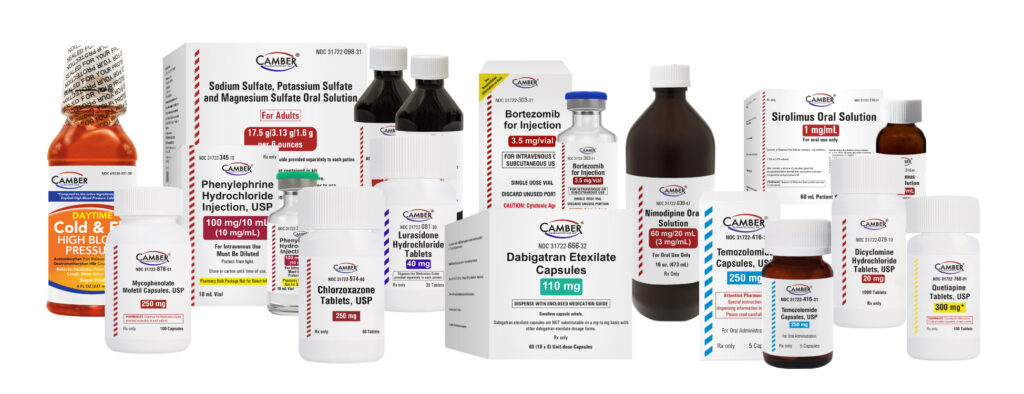
Products Introduced in Q1 2025:
- Phenylephrine for Injection (generic for Vazculep®)
- Mycophenolate Mofetil Capsules (generic for CellCept®)
- Dabigatran Capsule Blister Packs (generic for Pradaxa®)
- Chlorzoxazone Tablets (generic for Paraflex®)
- Temozolomide Capsules (generic for Temodar®)
- Sodium Sulfate-Potassium Sulfate-Magnesium Sulfate Oral Solution (generic for Suprep® Bowel Prep Kit)
- Lurasidone HCl Tablets (generic for Latuda®)
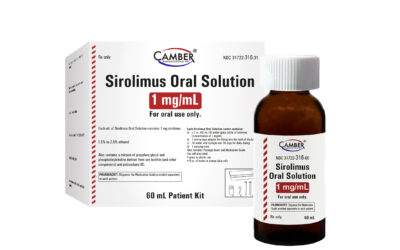
- Sirolimus Oral Solution (generic for Rapamune®)
- Dicyclomine HCl Tablet (generic for Bentyl®)
- Bortezomib Injection (generic for Velcade®)
- Nimodipine Oral Solution
- Quetiapine Tablets (generic for Seroquel®)
- Daytime Cold & Flu HBP (generic for DayQuil HBP Cold & Flu®)
Founded in 1993, Hetero is India’s largest privately held pharmaceutical company and a global leader in Active Pharmaceutical Ingredients (APIs) and Finished Dosage Forms (FDFs). With 36 cutting-edge manufacturing facilities worldwide, Hetero continues to drive innovation and excellence across global healthcare markets. For more information about these new launches or our expanding pipeline, please contact your Camber representative.
Recent articles
- Camber Launches 13 New Generic Products in Q1 2025, Sets Ambitious Pace for the Year
- Camber Launches Mycophenolate Mofetil Capsules
- Camber Launches Sodium Sulfate, Potassium Sulfate and Magnesium Sulfate Oral Solution
- Camber’s Triple Play – Featured Generics
- Big Game Commercial
- Charting a Bold Path for 2025